Chemistry of Colors

Vincent van Gogh's Bedroom in Arles is a colorful mix of bright orange, red, blue, and green and expresses the 'absolute restfulness' of his bedroom.
Table of Contents
“Colors are the smiles of Nature.” - Leigh Hunt
…and true colors are the smiles of chemistry.
A Brief History of Colors
Colors have been used before writing was invented. Over 200 thousand years ago, early humans ground up rocks and minerals like hematite, also known as iron (iii) oxide for cave paintings and object decoreation. Hematite contains Fe3+ ions, which have a reddish-brown color. Early humans began doing primitive chemistry to create other colors. Adding water to hematite created limonite, a mixture of multiple minerals that has a yellow color.

One of the first synthetic pigments humans made was Egyptian Blue. As the name suggests, it was made by early Egyptians around 5000 years ago. Blue-colored substances are rare in nature, so Egyptian blue allowed for a synthetic substitute for obtaining a blue color. Egyptian blue is made by heating a ground-up mixture of sand, chalk, a source of copper, and embalming salts to about 900oC for 10 to 100 hours!
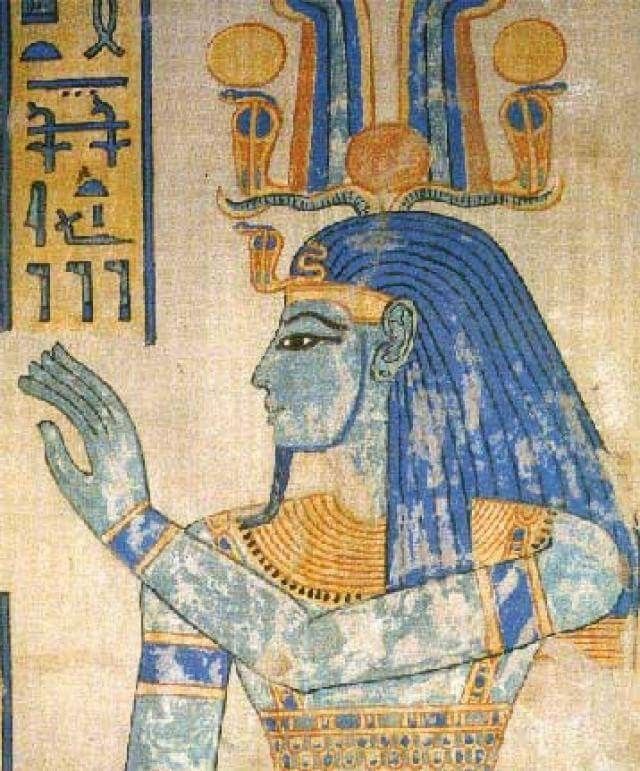
Jumping ahead to the 19th century, many famous artists like Vincent van Gogh came to prominence. To create paintings, artists used paints that were, and still are, created by mixing a dried and ground-up pigment, a solvent to make the paint liquid, and a binder that keeps the pigment and solvent from separating. Vincent van Gogh's famous painting at the beginning of this post uses a multitude of compounds to create color. Black was made from crushed sources of carbon such as charred bones, white was made from lead salts (which we now know are toxic), red was made from toxic mercury compounds, blue was made from iron compounds, yellow was made from a mixture of lead and chromium, and a deep green was made from a mixture of copper and arsenic (arsenic being toxic, many artists, including Vincent van Gogh, grew insane from arsenic poisoning).
Further research: PBS Studios - The Chemistry of Color
The Basics of Color
Before we can understand how chemistry plays a role in coloring compounds, we must learn why colors exist in the first place.
Our eyes see a range of light called the visible spectrum; light outside of the visible spectrum range is invisible to our eyes. When an object has a color, it means that the object does not absorb, but reflect that color. For example, a red book does not absorb red light, so red light reflects off the book and reaches our eye, making the book look red.
Therefore, if an object absorbs a color, the object’s color will be the color opposite to the absorbed color on the color wheel (two colors that are opposite to each other on the color wheel are called complementary colors).
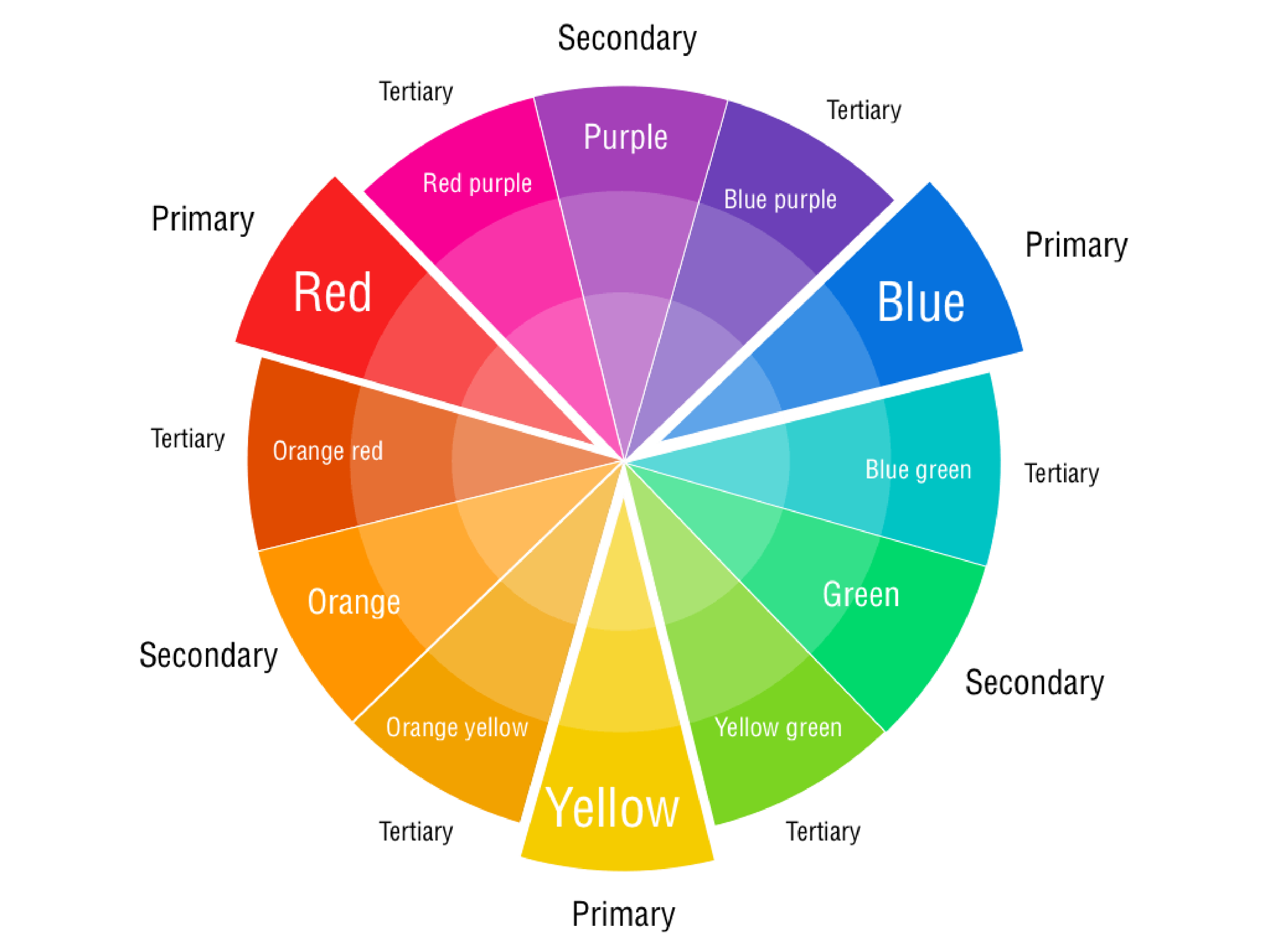
Based on the color wheel above, an object that absorbs orange light will look blue, and an object that absorbs blue-green light will have an orange-red look. Transparent objects do not reflect light, but instead let unabsorbed light pass through itself. Black objects absorb almost all visible light, and white objects reflect almost all visible light.
The Organic Side of Colors
So what gives compounds their color?
Lycopene (a red pigment found in tomatoes), zeaxanthin (an orange-red compound found in wolfberries), lutein (a yellow compound found in egg yolks), chlorophyll b (a green compound found in plants), indigotin (a blue compound in indigo dye), and petunidin (a purple compound found in some grapes) are organic compounds whose colors range throughout the visible spectrum.
The cause for the variety of color? Conjugated pi bonds.
Pi bonds are in double and triple bonds in molecules. Conjugated pi bonds mean that there is an alternating pattern of double/triple bond, single bond, double/triple bond, single bond, etc.

Molecule of Beta Carotene showing an alternating pattern of 11 double bonds (conjugated pi bonds). It is orange-red in color and found in carrots.
Compounds with a large continuous conjugated pi bond system generally absorb light in the visible spectrum. The larger the conjugated pi bond system, the longer the wavelength of light that is absorbed.
This is because each pi bond has two energy levels: one in a bonding orbital and the other in an antibonding orbital. Each orbital holds up to two electrons, with the lower energy levels getting filled up before the higher energy levels. The bonding orbitals are the lower energy levels, while the antibonding orbitals are the higher energy levels.
Thus, when there are n conjugated pi bonds in a conjugated pi bond system, there exist 2n electrons in the conjugated pi bond system, n bonding orbitals in the conjugated pi bond system, and n antibonding orbitals in the conjugated pi bond system. In the image below, antibonding orbitals are marked with a *
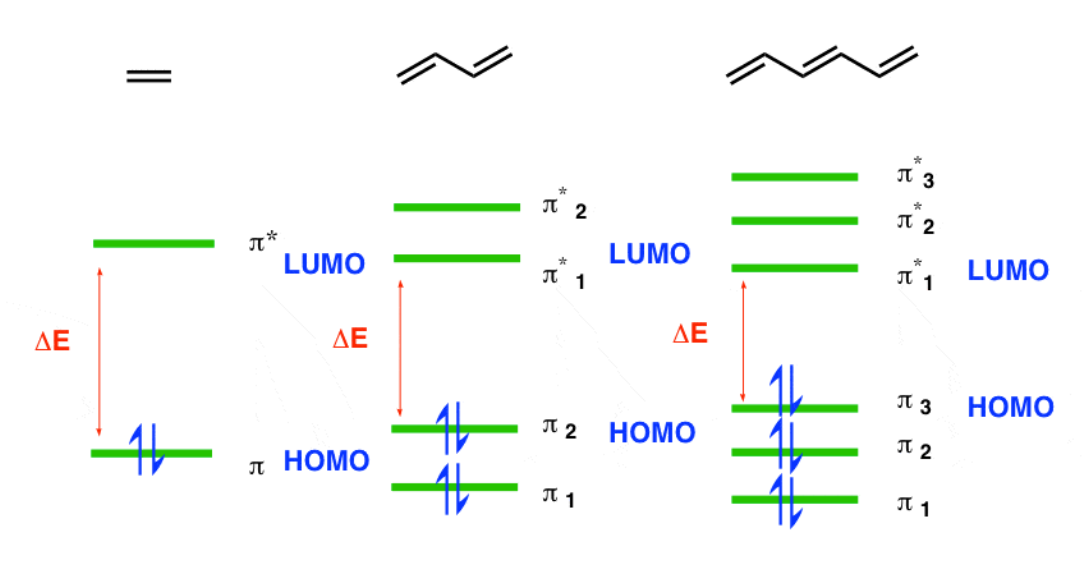
HOMO = Highest Occupied Molecular Orbital, LUMO = Lowest Unoccupied Molecular Orbital
The wavelength of light increases going from violet to red to light. As the wavelength of light increases, the energy of the light decreases.
Usually, the conjugated pi bond system must have more than eight continuously conjugated pi bonds to go from absorbing UV light to visible light, producing a visible color.
How about some examples?
Isophorone (Colorless)
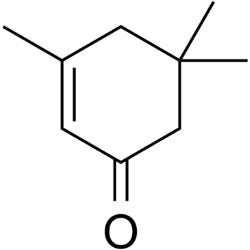
Isophorone has a conjugated pi bond system of only two conjugated pi bonds. It is colorless.
Lycopene (Red)
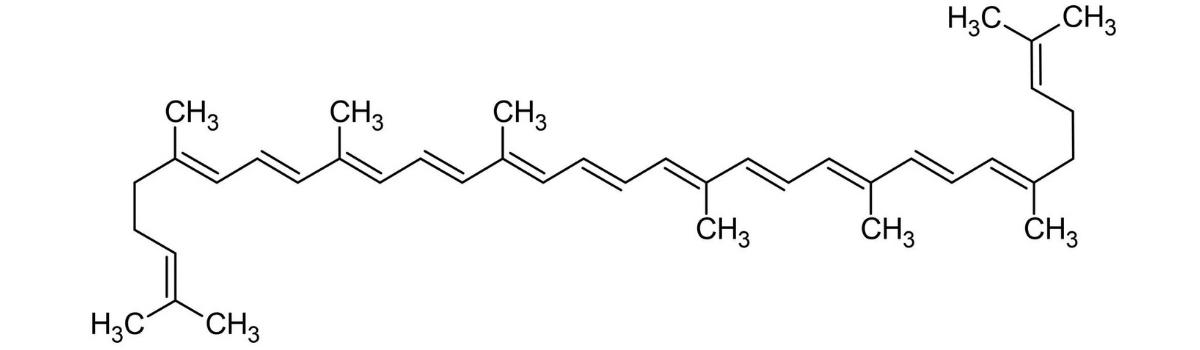
Lycopene has a conjugated pi bond system of 11 conjugated pi bonds. It looks red because it absorbs green light; green and red are complimentary colors.
Chlorophyll b (Green)
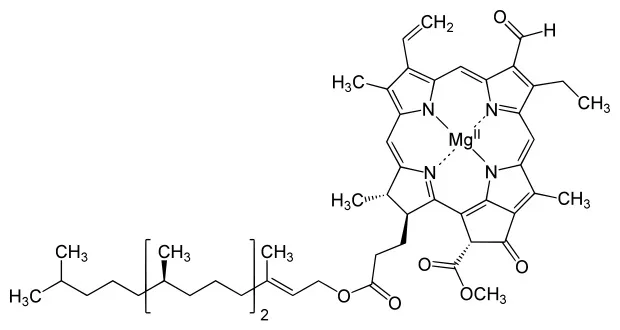
Chlorophyll b has a conjugated pi bond system of 13 conjugated pi bonds. It looks green because it absorbs red light.
Zeaxanthin (Yellow-Orange)
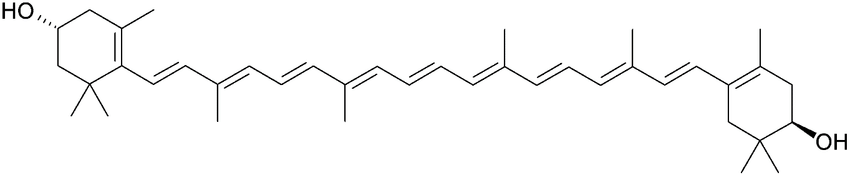
Zeaxanthin has a conjugated pi bond system of 11 conjugated pi bonds. It looks yellow-orange because it absorbs blue-violet light.
Hold on a second! Both lycopene and zeaxanthin have a conjugated pi bond system of 11 conjugated pi bonds, so why do they have different colors?
Well, the number of conjugated pi bonds in a conjugated pi bond system is not the only factor that determines the color of organic compounds. Whether the conjugated pi bonds are attached to different substituents/functional groups and the location of the conjugated pi bonds also affects the wavelength absorbed.
Nevertheless, determining the number of conjugated pi bonds in a conjugated pi bond system is the primary factor in determining the color of most organic compounds.
Fun fact: Bleach reacts with colored organic compounds and destroys double bonds, causing the color of the compound to disappear or lighten. This is the reason why bleach gets rid of color in clothes. Your clothes still stay stained, however the stain simply no longer shows up.